Pharmaceutical
Our expertise
-
- Information Technology
- Industrial Internet of Things (IIoT)
- Cloud Computing
- Electronic Batch Record (EBR)
-
Formulation
Formulation is the process in which different chemical substances, including the active drug are combined to produce a final medicinal product.
Eliminating paperwork, reducing errors and maintaining high quality standards will increase the throughput of the plant
-
- • Improve consistency
- • Increase throughput
- • Reduce waste
- • Improve product quality
- • Reduce compliance cost
- • Reduce inventory levels
- • Reduce Cycle time
- • Improve customer service
- • Maximize return on net assets
-
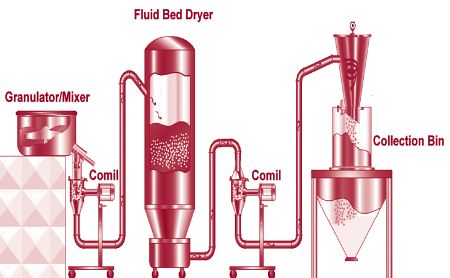
- • Rapid Mixer Granulator
- • Fluid Bed Dryer
- • Blender
- • Vacuum Tray Dryer
- • Tablet Compression
- • Auto Coater
- • Strip Packing
- • Powder Filling
- • CIP/SIP System
-
Active Pharmaceutical Ingredients
API can be defined as the chemicals used to manufacture pharmaceuticals drugs
- • Bulk Chemical: Large volume production & Lower unit cost
- • Fine Chemical: Smaller scale & relatively higher cost
-
Benefit of Automation :
- • Minimize people intervention
- • Maintain accurate and reproducible operating conditions for reaction
- • Integration of multiple operations
-
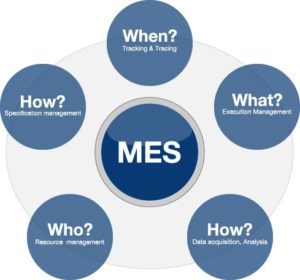
Manufacturing Execution Systems
- • Paper-based processes are replaced as MES software manages master batch records and electronic batch recording
- • The MES system covers the entire manufacturing process from product dispensing and weighing, tableting and filling of liquids, through to packaging
- • EBR helps ensure the batch documentation is correct and comprehensive, with all processes and specifications enforced
- • Retrieving data from machines or line controllers into the batch record, which allows eliminating error and capturing more data
Chemical
- Sulphur Recovery
- Secondary Brine Processing
- LNG Train
- Conveyor Systems
- Lube Plant
- Hydrogen Plant
- Co2 Plant
- Ammonia Plant
- Fertilizers
- Distillation